

Lower energy sub-shells are closer to the nucleus.Different sub-shells have different shapes. There are 4 different sub-shells – s, p, dand f. Well, electrons in each shell can be further split into mini sub-shells, meaning that the electrons in the same shell do not all have the same energy level. We learnt above that electrons are split into shells, each with their own energy level. Electron shells are divided into sub-shells which each have a different energy.So the further away a shell is from the nucleus, the higher the principle quantum number. The shell closest to the nucleus has quantum number 1, the shell second closest has quantum number 2, and so on. Each electron shell has a principal quantum number. Principal quantum number is the number given to each electron shell.The energy level of electron shells increases as distance from the nucleus increases, as this means there is a decreased interaction with the nucleus. An electron shell that is further from the nucleus has a higher energy level.The fixed energy of an electron is the amount of energy needed to move electrons from one energy level to another. Each electron shell has a particular energy, and electrons can only have one of th e fixed energy levels of the shells – they cannot have intermediate energy values that don’t lie on one of the energy levels. The shells are found at different distances from the nucleus, so electrons in different shells have different energy levels. Instead, they are found in different shells, which represent specific energy levels.
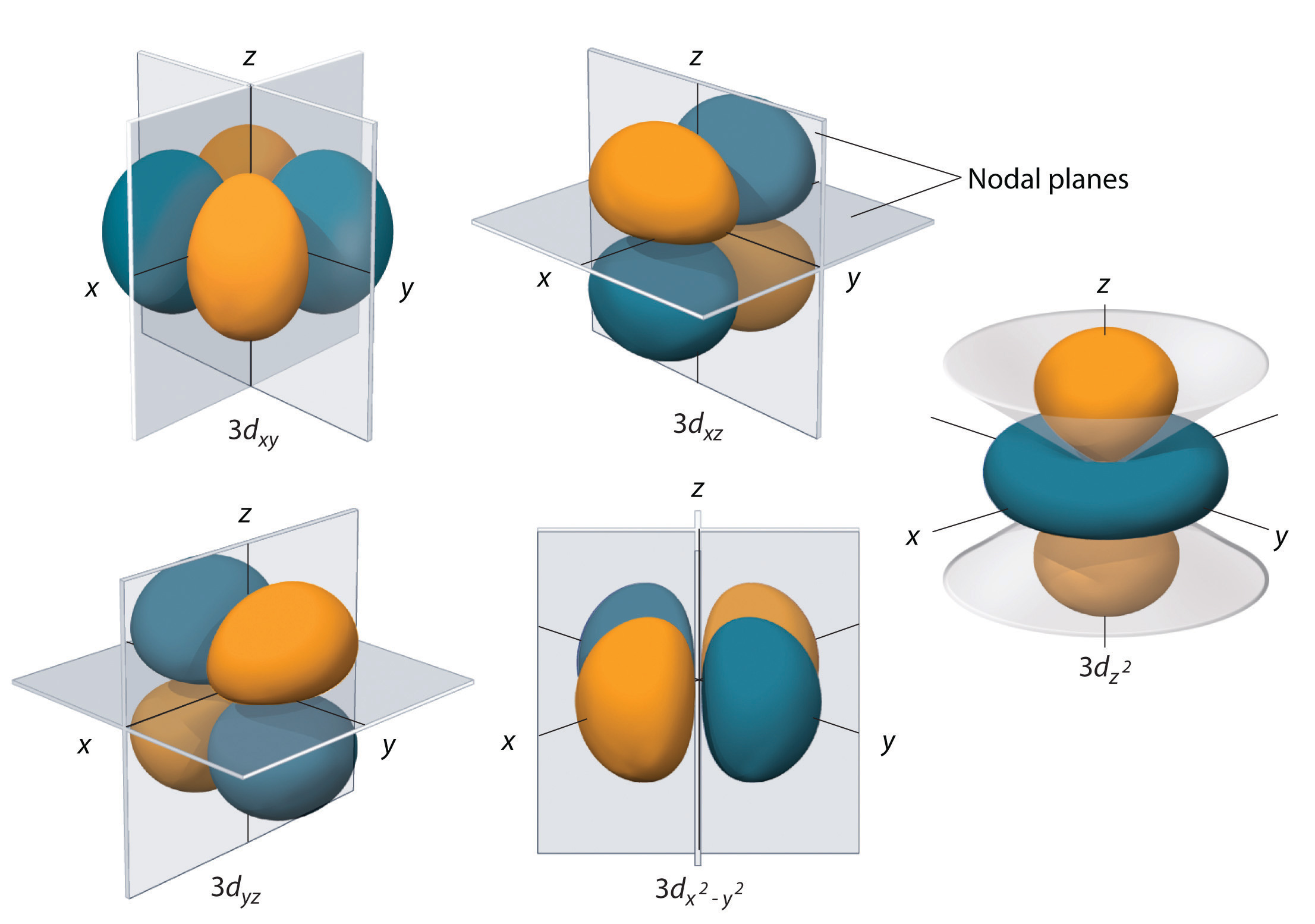
When electrons move around the nucleus, they don’t all move in one orbit. Electrons move in electron shells around the central nucleus.Atomic Structure - Electrons in Atoms (A-Level Chemistry) Electron Shells and Sub-Shells DefinitionsĮlectron shells are regions of space at different distances from the nucleus which can contain up to a certain number of electrons of a fixed energy level.Ī sub-shell is one or more orbitals in the same shell which have the same energy levels.Īn orbitalis the volume of space where an electron is most likely to be found.


 0 kommentar(er)
0 kommentar(er)
